Lung ultrasound and assessment of fluid overload in peritoneal dialysis
DOI:
https://doi.org/10.25796/bdd.v8i3.87083Keywords:
B-lines, Lung Ultrasonography, Peritoneal dialysis, Subclinical Hypervolemia, Volume StatusAbstract
Fluid management remains a major challenge in peritoneal dialysis (PD), particularly due to the difficulty in detecting subclinical hypervolemia. This cross-sectional, single-center study, with a sample of 22 patients, investigated the utility of lung ultrasound (LUS) as a complementary tool for volume status assessment. Using an 8-zone scanning protocol and B-line scoring system, LUS was performed during routine visits alongside inferior vena cava (IVC) evaluation and bioimpedance analysis (BIA). The results showed strong correlations between B-line scores and both overhydration, as measured by BIA (r = 0.625), and IVC collapsibility index (r = –0.722). Notably, half of the patients considered clinically euvolemic exhibited signs of hypervolemia based on ultrasonographic criteria. These findings suggest that LUS is a noninvasive, practical, and effective method for identifying fluid overload not evident through standard clinical evaluation, supporting its integration into routine care for PD patients.
INTRODUCTION
Estimation of ideal volume status is a constant challenge in nephrology. This is usually based on clinical evaluation, along with other diagnostic tools such as natriuretic peptides and bioimpedance spectroscopy (BIS). Several methods have been developed to accurately assess fluid status in dialysis patients, including BIS, measurement of inferior vena cava (IVC) diameter by ultrasound, analysis of biomarkers such as natriuretic peptides, and, more recently, the observation of lung comets using chest ultrasound [1].
In 1996, Dr. Daniel Lichtenstein first described the comet tail artifact detected in lung ultrasound in a cohort of patients with both cardiogenic and noncardiogenic pulmonary edema [2]. This artifact is caused by micro-reflections at the subpleural interface, which the ultrasound interprets as distance, caused by the presence of extravascular lung water or small air-fluid interfaces, resulting in a narrow, laser-like ray on the screen. The artifact, now known as the B-line, has contributed to our ability to detect both clinical and, especially, subclinical pulmonary edema, offering a more precise and accessible alternative to traditional methods such as lung auscultation or chest radiography [3]. One of its main limitations is the lack of specificity. B-lines are a sonographic sign of lung interstitial syndrome, but they cannot differentiate between thickening of the interlobular septa caused by excess lung water and fibrotic thickening, as seen in pulmonary fibrosis (dry B-lines). Therefore, caution must be exercised when interpreting LUS findings in patients with known or suspected pulmonary fibrosis [4].
Many studies have demonstrated the clinical use of LUS. Studies such as LUS-HF and CLUSTER-HF have shown the use of LUS in guiding more intensive diuretic treatment in heart failure patients, leading to a reduction in B-lines and improved management of fluid overload[5][6]. While there are few studies on subclinical hypervolemia in chronic kidney disease (CKD) patients, one study by Baki et al. demonstrated that the combination of elevated natriuretic peptide levels and decreased inferior vena cava collapsibility index (IVCCI) increased the specificity and positive predictive value for detecting subclinical hypervolemia [7].
It is well known that the prevalence of hypervolemia in PD patients is considerable and is associated with increased mortality [8]. Persistent volume overload increases arterial stiffness, causes higher systolic BP, lower diastolic BP, and increased pulse pressure. This increases afterload to the left ventricle, resulting in left ventricular hypertrophy (LVH). All of these effects have been identified as independent risk factors for cardiovascular morbidity and mortality in end-stage kidney disease (ESKD) [9][10].
Asymptomatic lung congestion has been described in a significant proportion of dialysis patients [11]. In a multicenter study by Zoccali et al., moderate to severe lung congestion was present in 45% of hemodialysis patients, with 71% of these patients being asymptomatic or presenting only slight symptoms of heart failure [12]. Another study by Enia et al. reported that 58% of hemodialysis patients exhibited moderate to severe lung congestion, 38% of whom were asymptomatic (NYHA class I) [13]. Additionally, Mallamaci et al. demonstrated that 57% of asymptomatic dialysis patients exhibited moderate to severe lung congestion [14]. Thus, some dialysis patients are erroneously considered euvolemic.
Increased precision in volemia evaluation is an important unmet need in dialysis patients, particularly PD patients. Some studies suggest that PD patients may be more prone to overhydration compared to those undergoing hemodialysis (HD). Achieving euvolemia in PD patients is especially challenging because PD depends on self-treatment, daily ultrafiltration, and residual renal function, all of which can vary widely among individuals [8][15][16].
LUS has been validated as an effective method for evaluating extracellular lung water, particularly in heart failure and hemodialysis patients [1][16]. Although its role in PD patients remains uncertain, it may play an important role in evaluating volume status in PD patients. In hemodialysis, LUS has demonstrated a strong prognostic value, as the number of B-lines correlates with adverse cardiovascular outcomes and mortality, and LUS-guided ultrafiltration can safely reduce pulmonary congestion without increasing intradialytic complications. In peritoneal dialysis, LUS findings do not consistently correlate with bioimpedance or clinical signs. This suggests that LUS and other assessment methods evaluate different fluid compartments and are therefore complementary [1][11][15][17].
The technique itself is cost-effective and widely accessible, requiring only 3-5 minutes to perform. It can be efficiently carried out by trained technicians and demonstrates low interobserver variability [18]. The 8-point scoring system has shown excellent diagnostic performance in the evaluation of ESKD patients [19][20]. Measurement of IVCCI adds information about intravascular volume status [21].
Given these considerations, combining different methods and technologies may improve the detection of subclinical hypervolemia in PD patients. As such, we aimed to estimate its prevalence using LUS and to determine the concordance between LUS findings, IVCCI, and other clinical parameters and tools, such as BIS.
METHODS
Patient Selection and Recruitment
This study was approved by the local ethics committee. All participants were provided informed consent.
This was an opportunistic, cross-sectional study involving peritoneal dialysis (PD) patients during routine clinic visits. Patients were not pre-selected based on specific characteristics but were included if they met the following criteria: duration on PD greater than 3 months, absence of active infections, and willingness to participate. Recruitment occurred during routine outpatient visits in the first semester of 2023 and was dependent on the availability of both staff and equipment to perform the assessments. All eligible patients who attended during the study period, and for whom logistical conditions allowed (i.e., availability of human and technical resources), were invited to participate. There was no subjective or selective inclusion process; rather, patients were enrolled consecutively whenever conditions permitted.
Demographics
Data regarding age, sex, cause of CKD, past medical history of smoking, and comorbidities such as diabetes, heart failure, or lung disease were extracted from the patient's medical records.
Clinical Evaluation
Upon visit, patients were questioned about symptoms of hypervolemia: orthopnea and dyspnea. A physical examination was performed, and data regarding weight, blood pressure, presence of crackles on pulmonary auscultation, and presence of pretibial edema were noted.
Laboratory Data
Laboratory parameters performed for the last PD visit were accessed. The last serum values for NT-proBNP, sodium, chloride, bicarbonate, and albumin were recorded.
Imaging Studies
Most patients had undergone chest radiograph and echocardiogram within the past year. The presence or absence of pleural effusion on chest radiograph was noted. Ultrasonographic data on left atrium diameter, left ventricular ejection fraction, left ventricular mass index, and the presence or absence of pericardial effusion were collected.
Bioimpedance Analysis
Bioimpedance analysis with Body Composition Monitor (Fresenius Medical Care) was routinely performed during each PD consult. The last bioimpedance analysis data regarding hydration status were recorded for each patient on the same day.
Ultrasound Evaluation
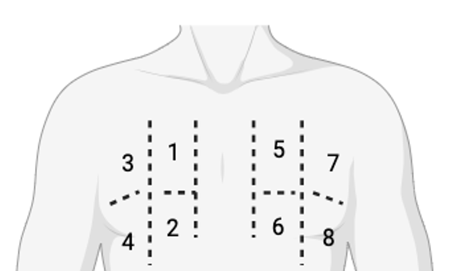
We performed ultrasound during routine PD visits, evaluating LUS using an 8-site scanning semiquantitative protocol. Four sites in each hemithorax were scanned (Figure 1). Each site (1 to 8) was scored according to Table I. Both the B-line score and the total number of B-lines for each site were recorded.
Figure 1.8-site scanning protocol. Each hemithorax is divided vertically by the midclavicular line and horizontally by the 3rd intercostal space
| Lung Ultrasound Pattern | Score |
|---|---|
| Normal A-Line pattern or <3 lines/space | 0 |
| Well separated B-lines (≥3 per rib space) | 1 |
| Coalescent B-lines (≥3 per rib space) | 2 |
| >10 B-lines/Consolidation | 3 |
Inferior vena cava (IVC) evaluation was performed during routine visits in order to calculate IVC Collapsibility Index (IVCCI). IVCCI was defined as in Figure 2 below.
Figure 2.Collapsibility index (IVCCI) calculation. dMax: maximum inferior vena cavq diameter ; dMin: minimum inferior vena cava diameter
Operator Training and Interobserver Reproducibility
Lung ultrasound and IVC assessments were performed by two operators who completed a dedicated pont-of-care ultrasound (POCUS) training course for nephrologists and participated in a one-week practical internship at a well-recognized center, where regular evaluation of B-lines and IVC measurements is performed. This training aimed to ensure consistency and reliability of the echographic assessments.
Definition of Subclinical Hypervolemia
In the absence of standardized criteria for diagnosing subclinical hypervolemia in the medical literature, we adopted an exploratory echographic approach combining LUS findings and the IVCCI. Subclinical hypervolemia was defined as the absence of overt clinical signs of fluid overload, alongside a total Bline score ≥4 and/or IVCCI <50%. While B-lines are established markers of extravascular lung water, and IVCCI <50% suggests volume overload, the specific thresholds used here are not yet standardized or universally validated. This combination of echographic parameters aims to improve diagnostic accuracy but should be considered a pragmatic, hypothesis-generating approach, pending further validation.
Statistical Analysis
Categorical variables are presented as frequencies and percentages, and continuous variables as means and SD, or medians and interquartile ranges for variables with skewed distributions. Normal distributions were checked by evaluating skewness and kurtosis. All reported p-values are two-tailed with a p-value of 0.05 indicating statistical significance. Correlation analysis was done using the Pearson correlation test, with corresponding 95% confidence intervals (CI) reported. Analyses were performed using SPSS Statistics, version 25.
RESULTS
Twenty-two patients were enrolled. The mean age was 61 ± 11 years, and 59% were male. The median time on PD was 30 months (Table II). The prevalence of diabetes mellitus was similar across the groups. Regarding technique-related variables, 27% of the total cohort were on automated peritoneal dialysis (APD), with a slightly higher proportion (40%) in the higher B-line group. Notably, only five patients had a B-line score ≥4.
|
Total (n = 22) |
Score <4 (n = 17) |
Score ≥4 (n = 5) |
p-value | |
|---|---|---|---|---|
| Age – mean ± SD | 61 ± 11 | 60 ± 12 | 57 ± 4 | 0.251 |
| Male – n (%) | 13 (59) | 9 (53) | 4 (80) | 0.360 |
| Comorbidities – n (%) | ||||
| Heart Failure | 4 (18) | 4 (24) | 0 | / |
| Diabetes Mellitus | 5 (23) | 4 (24) | 1 (20) | 0.687 |
| Smoking History | 4 (18) | 3 (21) | 1 (20) | 0.489 |
| Pulmonary Disease | 2 (9) | 2 (11) | 0 | / |
| Technique Related Variables | ||||
| Time in PD (months) – median (Q3–Q1) | 30 (53–12) | 34 (55–18) | 12 (31–4) | 0.085 |
| Automated PD – n (%) | 6 (27) | 4 (24) | 2 (40) | 0.535 |
| Icodextrin Use – n (%) | 16 (72) | 12 (71) | 4 (80) | 1 |
| RRF – mean ± SD | 1,334 ± 887 | 1,300 (1,600–1,050) | 1,000 (1,650–100) | 0.233 |
| Ultrafiltration – mean ± SD | 711 ± 513 | 615 ± 413 | 1,086 ± 697 | 0.07 |
Residual renal function (RRF) tended to be lower in patients with higher B-line scores (mean of 1,334 ± 887 mL in the total cohort vs. 1,000 mL in the higher B-line group). Patients with higher B-line scores also had significantly higher mean diastolic blood pressure (75 ± 11 vs. 89 ± 15 mmHg, p = 0.029) and lower serum albumin levels (4.1 ± 0.3 vs. 3.7 ± 0.5 g/dL, p = 0.032), as detailed in Table III
|
Total (n = 22) |
Score <4 (n = 17) |
Score ≥4 (n = 5) |
p-value | |
|---|---|---|---|---|
| Clinical Data/Physical Exam | ||||
| Dyspnea (yes) – n (%) | 2 (9) | 1 (6) | 1 (20) | 0.411 |
| Crackles on Lung Auscultation (yes) – n (%) | 3 (14) | 2 (12) | 1 (20) | 0.700 |
| Peripheral Edema (yes) – n (%) | 3 (14) | 3 (14) | 0 (0) | / |
| Systolic BP – (mmHg) mean ± SD | 135 ± 22 | 132 ± 20 | 143 ± 29 | 0.347 |
| Diastolic BP – (mmHg) mean ± SD | 78 ± 13 | 75 ± 11 | 89 ± 15 | 0.029 |
| Laboratory Results | ||||
| NT-ProBNP (pg/ml) – median (Q3–Q1) | 1,143 (5,323–668) | 861 (4,243–649) | 4,141 (16,513–980) | 0.225 |
| Serum albumin (g/dL) – mean ± SD | 4 ± 0.4 | 4.1 ± 0.3 | 3.7 ± 0.5 | 0.032 |
| Serum Na (mmol/l) – mean ± SD | 138 ± 3 | 138 ± 3 | 139 ± 2 | 0.677 |
| Serum Cl (mmol/l) – mean ± SD | 98 ± 4 | 99 ± 4 | 98 ± 2 | 0.791 |
| Serum HCO3 (mmol/l) – mean ± SD | 34 ± 3 | 24 ± 4 | 24 ± 4 | 0.581 |
| Bioimpedance Spectroscopy | ||||
| Overhydration (L) – mean ± SD | 0.8 ± 1.5 | 0.3 ± 1.3 | 2.3 ± 0.9 | 0.005 |
| Inferior Vena Cava Evaluation | ||||
| IVC dMax (mm) – mean ± SD | 14 ± 13 | 12 ± 4 | 15 ± 3 | 0.139 |
| IVC dMin (mm) – mean ± SD | 7 ± 4 | 8 ± 3 | 12 ± 5 | 0.066 |
| IVCCI (%) – mean ± SD | 40 ± 13 | 42 ± 12 | 31 ± 12 | 0.104 |
| Radiography | ||||
| Pleural Effusion – n (%) | 6 (27) | 4 (23) | 2 (40) | 0.585 |
| Echocardiography | ||||
| Pericardial Effusion – n (%) | 1 (5) | 0 | 1 (20) | / |
| LVEF – mean ± SD | 57 ± 9 | 56 ± 10 | 58 ± 7 | 0.681 |
| LA diameter (mm) – mean ± SD | 40 ± 6 | 40 ± 6 | 41 ± 6 | 0.685 |
| LVMI g/m2 – median (Q3–Q1) | 97 (122–80) | 100 (121–80) | 94 (140–74) | 0.845 |
| Medications | ||||
| Diuretics – n (%) | 18 (82) | 13 (77) | 5 (100) | 0.585 |
| Nº anti-HTA drugs – median (Q3–Q1) | 3 (3–1) | 3 (3–1) | 2 (4–1) | 0.869 |
Patients with higher B-line scores tended to have elevated hydration status, as assessed by BIA, and a lower IVCCI. Echocardiographic findings revealed no significant differences between patients with higher and lower B-line scores. Key parameters, such as left ventricular ejection fraction (LVEF), were preserved across groups, with values consistently within the normal range. No notable variations in cardiac chamber sizes or valvular function were observed between groups. Additionally, radiographic findings revealed that patients with a higher B-line score had a higher prevalence of pulmonary congestion, with chest X-rays showing signs of interstitial edema and mild pleural effusions.
All patients in the higher B-line score group were on diuretics, had higher ultrafiltration rates, and lower residual renal function, further underscoring the challenge in fluid management.
Concordance Between Fluid Overload Assessment Methods
We observed a positive correlation between B-line score and overhydration on BIA (r = 0.625, p = 0.002). Inverse correlations between B-line score and both IVCCI (r = –0.722, p = 0.001) and residual renal function (r = –0.628, p = 0.002) were observed. We did not identify a statistically significant correlation between B-line score and N-terminal brain natriuretic peptide (NT-proBNP) (r = 0.334, p = 0.129). These results are summarized in Table IV. Similar results were obtained using the total number of B-lines (as opposed to the scoring system).
| Parameter 1 | Parameter 2 | Correlation (r) | 95% CI [LL, UL] | p-value |
|---|---|---|---|---|
| B-line Score | Overhydration (BIA) | 0.625 | [0.276, 0.828] | 0.002 |
| B-line Score | IVCCI | –0.722 | [–0.893, –0.37] | 0.001 |
| B-line Score | RRF | –0.628 | [–0.83, –0.281] | 0.002 |
| B-line Score | NT-proBNP | 0.334 | [–0.102, 0.662] | 0.129 |
Prevalence of Subclinical Hypervolemia in Peritoneal Dialysis Patients
We conducted a sub-analysis to identify the prevalence of subclinical hypervolemia among PD patients. Six patients (27%) who exhibited overt signs and symptoms of hypervolemia, including dyspnea, peripheral edema, and crackles on lung auscultation, were excluded from this evaluation. Eight patients (50%) were classified as having subclinical hypervolemia based on echographic criteria, defined by a Bline score greater than 4 and/or an IVCCI of less than 50%.
Patients diagnosed with subclinical hypervolemia had a significantly higher mean overhydration level (1.45 ± 1.2 L) compared to those without subclinical hypervolemia (-0.37 ± 0.83 L; p = 0.008), indicating an elevated volume of fluid retention. These patients had also notably elevated serum NT-proBNP levels (p = 0.046), a biomarker commonly associated with fluid overload and cardiac stress. Moreover, their residual renal function (RRF) was significantly lower (p = 0.028), reflecting the potential challenges in fluid removal in this group of PD patients.
Despite these findings, there were no significant differences between the groups concerning other demographic and laboratory parameters.
Table V summarizes the key differences between patients with subclinical hypervolemia and those with normovolemia.
|
Subclinical Hypervolemia (n = 8) |
Normovolemia (n = 8) |
p-value |
|
|---|---|---|---|
| Male – n (%) | 5 (62.5) | 4 (50) | 0.614 |
| Age (years) – mean ± SD | 63.4 ± 9.9 | 58.7 ± 12.9 | 0.428 |
| PD Vintage (months) – mean ± SD | 54.88 ± 87.5 | 31 ± 24.44 | 0.798 |
| Systolic BP (mmHg) – mean ± SD | 140 ± 19.9 | 127.4 ± 20.2 | 0.229 |
| Diastolic BP (mmHg) - mean ± SD | 79.4 ± 9.4 | 71.5 ± 14.2 | 0.212 |
| Overhydration (L) – mean ± SD | 1.45±1.2 | –0.37 ± 0.83 | 0.008 |
| Sodium (mmol/l) – mean ± SD | 138.9 ± 1.8 | 137.9 ± 3.2 | 0.511 |
| Albumin g/dL – mean ± SD | 3.85 ± 0.46 | 4.05 ± 0.34 | 0.338 |
| NT-proBNP (pg/ml) – mean ± SD | 7,132.5 ± 7,588.1 | 1,990.4 ± 2,788.2 | 0.046 |
| Residual Renal Function (ml) – mean ± SD | 950 ± 594.62 | 1,487.5 ± 418.1 | 0.028 |
DISCUSSION
In this study, LUS proved to be a valuable tool for detecting subclinical hypervolemia in PD patients. Higher B-line scores (≥4) were associated with increased hydration status, elevated NT-proBNP levels, and reduced residual renal function, despite all patients being on diuretics. These findings highlight the need for more aggressive fluid management strategies, such as icodextrin and hypertonic solutions, in patients with diuretic resistance and declining RRF.
Strong correlations between B-line scores and BIA overhydration, as well as IVCCI, reinforce the sensitivity of LUS in detecting fluid overload. LUS and BIA provide complementary insights: LUS assesses extravascular lung water, while BIA evaluates total body water, extracellular water (ECW), and intracellular water excess [16]. Given its noninvasive nature and bedside applicability, LUS is particularly beneficial for patients with borderline cardiac function, recurrent heart failure episodes, or increased ECW due to hypoalbuminemia.
However, a critical limitation of LUS is the specificity of B-lines, particularly in patients with chronic lung diseases, where "dry" B-lines may appear independently of fluid overload, potentially confounding interpretation [4]. In our cohort, none of the patients with a B-line score ≥4 had underlying chronic lung disease, which reduced this confounding factor in our findings. Nevertheless, cautious clinical correlation remains essential when applying LUS in populations with pulmonary comorbidities.
While our study was not designed for direct comparisons between LUS and other methods, the results support integrating B-line score assessments alongside BIA and NT-proBNP. The inverse correlation between B-line scores and IVCCI (r = -0.722) further confirms the relationship between pulmonary congestion and reduced venous collapsibility. Conversely, the low and nonsignificant correlation between B-line scores and NT-proBNP (r = 0.334, p = 0.129) emphasizes the limited discriminatory value of NT-proBNP alone in this context, likely due to multiple influencing factors, including residual renal function and comorbidities. Therefore, NT-proBNP should be interpreted cautiously and preferably in combination with other assessment tools [22] [23].
The clinical relevance of these findings lies in the early identification of fluid overload, particularly in patients with declining RRF (r = -0.628). LUS can provide real-time insights, enhancing patient awareness and compliance with fluid management strategies. Detection of subclinical hypervolemia could imply proactive changes in clinical practice, such as adjustments in ultrafiltration rates, initiation or intensification of icodextrin use, optimization of diuretic therapy, or reinforced patient education to improve adherence to fluid restrictions. Although some therapeutic adjustments were made based on the findings, these interventions were not standardized and, thus, were not systematically analyzed or included in this study. Future research should focus on implementing protocolized treatment modifications guided by a holistic approach, which should include LUS findings, to better assess their impact on renal survival, overall outcomes, and healthcare utilization in PD patients.
Importantly, this study is the first to evaluate subclinical hypervolemia in PD patients using LUS, emphasizing its feasibility, short learning curve, and high reproducibility. If an ultrasound device is available in the unit, its use should be maximized, as LUS protocols are simple and easy to implement in routine practice.
Despite its advantages, our study has limitations. The small sample size (n = 22) reduces statistical power and generalizability. Additionally, differences in clinical practices between centers and potential selection bias must be considered. The cost and availability of ultrasound equipment in some settings may also pose challenges. Moreover, while IVC measurements provide valuable volume status information, they are influenced by individual variability, tricuspid insufficiency, and intra-abdominal pressure changes due to PD [15]. Therefore, integrating with LUS with other markers is essential.
A common concern is the time required for additional assessments during routine visits. However, with adequate training, LUS can be performed quickly, especially using the 8-zone protocol, which significantly reduces procedure time compared to traditional methods. Given its practicality and clinical relevance, LUS should be incorporated into routine fluid management in PD patients to optimize care and improve outcomes.
CONCLUSION
Lung ultrasound is a promising, noninvasive, and highly reproducible tool for the early detection of subclinical fluid overload in PD patients. It effectively complements other methods, such as bioimpedance analysis and NT-proBNP levels, enabling a more comprehensive assessment of fluid status, particularly in patients with declining residual renal function. The findings of this study should be interpreted with caution, taking into account its small sample size, single-center design, and criteria used to define subclinical hypervolemia. All in all, LUS shows potential for broader clinical use. Future perspectives include conducting multicenter randomized studies to confirm the clinical impact of routinely integrating LUS into the follow-up of PD patients and to establish standardized protocols for its use in fluid management.
Contributions des auteurs
Joana Dias and Vitória Paes de Faria contributed to the conceptualization and design of the study. They collected the data, conducted the clinical assessments, performed the data analysis and contributed to the manuscript writing.
Ana Marta Gomes contributed to the conceptualization and data interpretation.
The manuscript was reviewed/interpreted and approved by all the other authors: Maria Beatriz Bessa; Susana Pereira, Daniela Lopes, Rute Carmo, João Carlos Fernandes, Maria Clara Almeida, and Ana Marta Gomes.
Ethical statement
This study was conducted in accordance with the principles of the Declaration of Helsinki. Ethical approval was obtained from the Ethics Committee of the Unidade Local de Saúde de Vila Nova de Gaia Espinho. All participants provided written informed consent prior to inclusion in the study.
Financial disclosure
The authors declare that they received no financial support or funding for the conduct of this study.
Conflits d’intérêts
The authors declare no conflicts of interest related to this work.
References
- Covic A., Siriopol D., Voroneanu L.. Use of Lung Ultrasound for the Assessment of Volume Status in CKD. American Journal of Kidney Diseases. 2018; 71:412-22. DOI
- Lichtenstein D., Mézière G., Biderman P., Gepner A., Barré O.. The Comet-tail Artifact. Am J Respir Crit Care Med. 1997; 156:1640-6. DOI
- Torino C., Gargani L., Sicari R., Letachowicz K., Ekart R., Fliser D.. The Agreement between Auscultation and Lung Ultrasound in Hemodialysis Patients: The LUST Study. Clinical Journal of the American Society of Nephrology. 2016; 11:2005-11. DOI
- Wang Y.K., Gargani L., Barskova T., Furst D.E., Cerinic M.M.. Usefulness of lung ultrasound B-lines in connective tissue disease-associated interstitial lung disease: A literature review. Arthritis Res Ther. 2017; 19DOI
- Rivas-Lasarte M., Álvarez-García J., Fernández-Martínez J., Maestro A., López-López L., Solé-González E.. Lung ultrasound-guided treatment in ambulatory patients with heart failure: a randomized controlled clinical trial (LUS-HF study. Eur J Heart Fail. 2019; 21:1605-13. DOI
- Araiza-Garaygordobil D., Gopar-Nieto R., Martinez-Amezcua P., Cabello-López A., Alanis-Estrada G., Luna-Herbert A.. A randomized controlled trial of lung ultrasound-guided therapy in heart failure (CLUSTER-HF study. Am Heart J. 2020; 227:31-9. DOI
- Wang Y., Cao X., Yu J., Zhang Y., Li X., Chen X.. Association of N-Terminal Pro-brain Natriuretic Peptide With Volume Status and Cardiac Function in Hemodialysis Patients. Front Cardiovasc Med. 2021; 8DOI
- Kim Y.-L., Van Biesen W.. Fluid Overload in Peritoneal Dialysis Patients. Semin Nephrol. 2017; 37:43-53. DOI
- AHCR Kamel, H Mansour. Are there any further modalities for prediction of subclinical volume overload in advanced stages of chronic kidney disease?. Kidney Res Clin Pract. 2021; 40:143-52. DOI
- Fornazarič D., Manja Antonič M., Knap B.. Volume status and arterial stiffness evaluation in peritoneal dialysis patients. Clin Nephrol. 2021; 96:74-9. DOI
- Sevinc M., Hasbal N.B., Basturk T., Ozcafer P.N., Kocas B.B., Kilickesmez K.. Comparison of lung ultrasound and other volumetric methods in peritoneal dialysis patients. Medicine. 2021; 100:e23856DOI
- Zoccali C., Torino C., Tripepi R., Tripepi G., D’Arrigo G., Postorino M.. Pulmonary Congestion Predicts Cardiac Events and Mortality in ESRD. Journal of the American Society of Nephrology. 2013; 24:639-46. DOI
- Enia G., Torino C., Panuccio V., Tripepi R., Postorino M., Aliotta R.. Asymptomatic Pulmonary Congestion and Physical Functioning in Hemodialysis Patients. Clinical Journal of the American Society of Nephrology. 2013; 8:1343-8. DOI
- Mallamaci F., Benedetto F.A., Tripepi R., Rastelli S., Castellino P., Tripepi G.. Detection of Pulmonary Congestion by Chest Ultrasound in Dialysis Patients. JACC Cardiovasc Imaging. 2010; 3:586-94. DOI
- Alexandrou M.-E., Balafa O., Sarafidis P.. Assessment of Hydration Status in Peritoneal Dialysis Patients: Validity, Prognostic Value, Strengths, and Limitations of Available Techniques. Am J Nephrol. 2020; 51:589-612. DOI
- Alexandrou M.-E., Theodorakopoulou M.P., Sarafidis P.A.. Lung Ultrasound as a Tool to Evaluate Fluid Accumulation in Dialysis Patients. Kidney Blood Press Res. 2022; 47:163-76. DOI
- Zoccali C., Torino C., Mallamaci F., Sarafidis P., Papagianni A., Ekart R.. A randomized multicenter trial on a lung ultrasound–guided treatment strategy in patients on chronic hemodialysis with high cardiovascular risk. Kidney Int. 2021; 100:1325-33. DOI
- Paudel K., Kausik T., Visser A., Ramballi C., Fan S.L.. Comparing lung ultrasound with bioimpedance spectroscopy for evaluating hydration in peritoneal dialysis patients. Nephrology. 2015; 20:1-5. DOI
- Torino C., Tripepi R., Loutradis C., Sarafidis P., Tripepi G., Mallamaci F.. Can the assessment of ultrasound lung water in haemodialysis patients be simplified?. Nephrology Dialysis Transplantation. 2021; 36:2321-6. DOI
- Buessler A., Chouihed T., Duarte K., Bassand A., Huot-Marchand M., Gottwalles Y.. Accuracy of Several Lung Ultrasound Methods for the Diagnosis of Acute Heart Failure in the ED. Chest. 2020; 157:99-110. DOI
- Kaptein M.J., Kaptein E.M.. Inferior Vena Cava Collapsibility Index: Clinical Validation and Application for Assessment of Relative Intravascular Volume. Adv Chronic Kidney Dis. 2021; 28:218-26. DOI
- Donadio C., Bozzoli L., Colombini E., Pisanu G., Ricchiuti G., Picano E.. Effective and Timely Evaluation of Pulmonary Congestion. Medicine. 2015; 94:e473DOI
- Curbelo J., Rodriguez-Cortes P., Aguilera M., Gil-Martinez P., Martín D., Suarez Fernandez C.. Comparison between inferior vena cava ultrasound, lung ultrasound, bioelectric impedance analysis, and natriuretic peptides in chronic heart failure. Curr Med Res Opin. 2019; 35:705-13. DOI
References
1. Covic A, Siriopol D, Voroneanu L. Use of Lung Ultrasound for the Assessment of Volume Status in CKD. American Journal of Kidney Diseases 2018;71:412–22. https://doi.org/10.1053/j.ajkd.2017.10.009.
2. Lichtenstein D, Mézière G, Biderman P, Gepner A, Barré O. The Comet-tail Artifact. Am J Respir Crit Care Med 1997;156:1640–6. https://doi.org/10.1164/ajrccm.156.5.96-07096.
3. Torino C, Gargani L, Sicari R, Letachowicz K, Ekart R, Fliser D, et al. The Agreement between Auscultation and Lung Ultrasound in Hemodialysis Patients: The LUST Study. Clinical Journal of the American Society of Nephrology 2016;11:2005–11. https://doi.org/10.2215/CJN.03890416.
4. Wang YK, Gargani L, Barskova T, Furst DE, Cerinic MM. Usefulness of lung ultrasound B-lines in connective tissue disease-associated interstitial lung disease: A literature review. Arthritis Res Ther 2017;19. https://doi.org/10.1186/s13075-017-1409-7.
5. Rivas-Lasarte M, Álvarez-García J, Fernández-Martínez J, Maestro A, López-López L, Solé-González E, et al. Lung ultrasound-guided treatment in ambulatory patients with heart failure: a randomized controlled clinical trial (LUS-HF study). Eur J Heart Fail 2019;21:1605–13. https://doi.org/10.1002/ejhf.1604.
6. Araiza-Garaygordobil D, Gopar-Nieto R, Martinez-Amezcua P, Cabello-López A, Alanis-Estrada G, Luna-Herbert A, et al. A randomized controlled trial of lung ultrasound-guided therapy in heart failure (CLUSTER-HF study). Am Heart J 2020;227:31–9. https://doi.org/10.1016/j.ahj.2020.06.003.
7. Wang Y, Cao X, Yu J, Zhang Y, Li X, Chen X, et al. Association of N-Terminal Pro-brain Natriuretic Peptide With Volume Status and Cardiac Function in Hemodialysis Patients. Front Cardiovasc Med 2021;8. https://doi.org/10.3389/fcvm.2021.646402.
8. Kim Y-L, Biesen W Van. Fluid Overload in Peritoneal Dialysis Patients. Semin Nephrol 2017;37:43–53. https://doi.org/10.1016/j.semnephrol.2016.10.006.
9. Baki AH, Kamel CR, Mansour H. Are there any further modalities for prediction of subclinical volume overload in advanced stages of chronic kidney disease? Kidney Res Clin Pract 2021;40:143–52. https://doi.org/10.23876/j.krcp.20.143.
10. Fornazarič D, Manja Antonič M, Knap B. Volume status and arterial stiffness evaluation in peritoneal dialysis patients. Clin Nephrol 2021;96:74–9. https://doi.org/10.5414/CNP96S13.
11. Sevinc M, Hasbal NB, Basturk T, Ozcafer PN, Kocas BB, Kilickesmez K, et al. Comparison of lung ultrasound and other volumetric methods in peritoneal dialysis patients. Medicine 2021;100:e23856. https://doi.org/10.1097/MD.0000000000023856.
12. Zoccali C, Torino C, Tripepi R, Tripepi G, D’Arrigo G, Postorino M, et al. Pulmonary Congestion Predicts Cardiac Events and Mortality in ESRD. Journal of the American Society of Nephrology 2013;24:639–46. https://doi.org/10.1681/ASN.2012100990.
13. Enia G, Torino C, Panuccio V, Tripepi R, Postorino M, Aliotta R, et al. Asymptomatic Pulmonary Congestion and Physical Functioning in Hemodialysis Patients. Clinical Journal of the American Society of Nephrology 2013;8:1343–8. https://doi.org/10.2215/CJN.11111012.
14. Mallamaci F, Benedetto FA, Tripepi R, Rastelli S, Castellino P, Tripepi G, et al. Detection of Pulmonary Congestion by Chest Ultrasound in Dialysis Patients. JACC Cardiovasc Imaging 2010;3:586–94. https://doi.org/10.1016/j.jcmg.2010.02.005.
15. Alexandrou M-E, Balafa O, Sarafidis P. Assessment of Hydration Status in Peritoneal Dialysis Patients: Validity, Prognostic Value, Strengths, and Limitations of Available Techniques. Am J Nephrol 2020;51:589–612. https://doi.org/10.1159/000509115.
16. Alexandrou M-E, Theodorakopoulou MP, Sarafidis PA. Lung Ultrasound as a Tool to Evaluate Fluid Accumulation in Dialysis Patients. Kidney Blood Press Res 2022;47:163–76. https://doi.org/10.1159/000521691.
17. Zoccali C, Torino C, Mallamaci F, Sarafidis P, Papagianni A, Ekart R, et al. A randomized multicenter trial on a lung ultrasound–guided treatment strategy in patients on chronic hemodialysis with high cardiovascular risk. Kidney Int 2021;100:1325–33. https://doi.org/10.1016/j.kint.2021.07.024.
18. Paudel K, Kausik T, Visser A, Ramballi C, Fan SL. Comparing lung ultrasound with bioimpedance spectroscopy for evaluating hydration in peritoneal dialysis patients. Nephrology 2015;20:1–5. https://doi.org/10.1111/nep.12342.
19. Torino C, Tripepi R, Loutradis C, Sarafidis P, Tripepi G, Mallamaci F, et al. Can the assessment of ultrasound lung water in haemodialysis patients be simplified? Nephrology Dialysis Transplantation 2021;36:2321–6. https://doi.org/10.1093/ndt/gfaa285.
20. Buessler A, Chouihed T, Duarte K, Bassand A, Huot-Marchand M, Gottwalles Y, et al. Accuracy of Several Lung Ultrasound Methods for the Diagnosis of Acute Heart Failure in the ED. Chest 2020;157:99–110. https://doi.org/10.1016/j.chest.2019.07.017.
21. Kaptein MJ, Kaptein EM. Inferior Vena Cava Collapsibility Index: Clinical Validation and Application for Assessment of Relative Intravascular Volume. Adv Chronic Kidney Dis 2021;28:218–26. https://doi.org/10.1053/j.ackd.2021.02.003.
22. Donadio C, Bozzoli L, Colombini E, Pisanu G, Ricchiuti G, Picano E, et al. Effective and Timely Evaluation of Pulmonary Congestion. Medicine 2015;94:e473. https://doi.org/10.1097/MD.0000000000000473.
23. Curbelo J, Rodriguez-Cortes P, Aguilera M, Gil-Martinez P, Martín D, Suarez Fernandez C. Comparison between inferior vena cava ultrasound, lung ultrasound, bioelectric impedance analysis, and natriuretic peptides in chronic heart failure. Curr Med Res Opin 2019;35:705–13. https://doi.org/10.1080/03007995.2018.1519502.
Downloads
Submitted
Accepted
Published
How to Cite
Issue
Section
License
Copyright (c) 2025 Joana Dias

This work is licensed under a Creative Commons Attribution 4.0 International License.






