Gastrostomy in an adult undergoing peritoneal dialysis: a case report
DOI:
https://doi.org/10.25796/bdd.v8i4.87092Keywords:
clinical case, peritoneal dialysis, gastrostomy, enteral nutrition, malnutritionAbstract
Malnutrition is a common complication in patients with chronic renal failure treated by peritoneal dialysis (PD), which can compromise prognosis. To control this malnutrition, feeding via a nasogastric tube may be necessary. However, in cases of insufficiency or intolerance, the placement of a gastrostomy may be an alternative. Its use in PD raises concerns about infection risk. We report the observation of a 67-year-old patient treated with continuous ambulatory peritoneal dialysis in whom a gastrostomy was performed in the context of severe malnutrition.
After starting PD in January 2020, the patient experienced several episodes of deterioration in his general condition and progressive malnutrition despite the introduction of oral nutritional supplements. Faced with worsening malnutrition (albumin 18.2 g/L), nasogastric feeding was attempted in October 2020 but withdrawn due to repeated disinsertions.
A gastrostomy was finally performed in March 2021 under antibiotic prophylaxis with cefazolin. PD was temporarily suspended in favor of hemodialysis and resumed without complications after healing. Reoperation was necessary after accidental removal of the tube, also without peritoneal complications. Tube feeding consisted of 1 L of high-calorie, high-protein solution (Mégaréal®) at night.
The patient’s nutritional status improved, with albumin rising from 25.8 g/L to 33 g/L.
The patient died suddenly in September 2021, unrelated to the gastrostomy or PD.
This observation illustrates the feasibility of gastrostomy in an adult on PD. Pediatric data show an acceptable risk of infection when a rigorous protocol is followed, including antibiotic prophylaxis, sometimes antifungal prophylaxis, and temporary PD adaptation. In our case, no peritonitis or leakage was observed after two gastrostomy placements.
This case illustrates the benefits of gastrostomy for the nutritional management of malnourished peritoneal dialysis patients, as well as the practical difficulties encountered.
Introduction
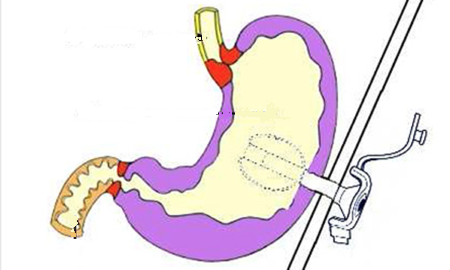
Malnutrition is a common complication and a major prognostic factor in patients with chronic renal failure undergoing peritoneal dialysis. Enriched oral nutrition may prove insufficient in cases of severe malnutrition, requiring enteral nutrition via nasogastric tube or gastrostomy.
In peritoneal dialysis patients, the placement of a gastrostomy raises questions about infection risk and its impact on the technical survival of dialysis. We report here a case of gastrostomy in a patient on peritoneal dialysis.
Clinical observation
Sixty seven years sixty-seven-year-old patient, retired, with a history of hypertension complicated by an intraparenchymal hematoma, asbestosis, and chronic renal failure of undetermined origin, was placed on continuous ambulatory peritoneal dialysis in January 2020.
On January 10, 2020, a Tenckhoff catheter was inserted by laparotomy under neuroleptanalgesia combined with local anesthesia. The procedure was performed without complications. Peritoneal dialysis (PD) was started on day 17 with a regimen consisting of Physioneal® 3.86% (2.5 L at 8 a.m.) and Physioneal® 1.86% (2.5 L at 12 p.m. and 4 p.m.), with an empty stomach at 8 p.m. The decision to initially use a hypertonic solution was based on recurrent lower-limb edema, suggesting significant fluid overload. This clinical context motivated the chosen therapeutic approach. A local Staphylococcus aureus infection quickly developed and was treated with Bactroban® for 8 days. The patient's starting weight was 82.6 kg, and his weight at discharge from the hospital was 83 kg. Pre-dialysis tests showed severe renal failure, as evidenced by an estimated glomerular filtration rate of 10 mL/min, elevated urea at 1.69 g/L, creatinine at 52.3 mg/L, albumin at 41.6 g/L, and prealbumin at 0.34 g/L. No oral nutritional supplements were introduced at this stage.
In May 2020, the patient was hospitalized for dehydration and diarrhea. The diuretic treatment was adjusted, and the PD regimen was modified to 3 exchanges of Physioneal® 1.36% (2.5 L), and empty peritoneum at night. The patient's weight at discharge was 87.6 kg.
In June 2020, hospitalization was scheduled for a fibroscopy and colonoscopy. Severe esophagitis and bulbitis were observed, and 10 colonic polyps were removed. The patient remained hospitalized for peritonitis that developed subsequently, with no identifiable pathogen. His weight at that time was 84 kg. Before considering nutritional management while maintaining peritoneal dialysis, it is essential to ensure that the patient is receiving adequate dialysis and is not in a state of underdialysis [1]. The biological evolution, marked by a clear decrease in urea to 0.52 g/L and stable creatinine at 53.7 mg/L, demonstrates the effectiveness of dialysis treatment. Given an albumin level of 28.6 g/L and a prealbumin level of 0.23 g/L, two Fresub-in® Max nutritional supplements were introduced.
In September 2020, another hospitalization was necessary after a fall at home with deterioration in general condition, accompanied by diarrhea and vomiting. Weight was 79 kg, albumin was 30.6 g/L, and prealbumin was 0.18 g/L. The two oral nutritional supplements were continued.
In October 2020, the patient was hospitalized for deterioration in his general condition, anorexia, and an infectious syndrome. Private nurses reported excessive alcohol consumption at home. Severe malnutrition was observed (albumin 18.2 g/L and prealbumin 0.15 g/L), and enteral nutrition was initiated via nasogastric tube with 1 L/24 h of Mégaréal®. His weight was 79.7 kg.
On November 18, 2020, the nasogastric tube was removed following repeated disinsertions. The patient refused to have the nasogastric tube reinserted or to undergo the proposed gastrostomy. The two oral nutritional supplements were resumed.
Between February and April 2021, the patient was hospitalized again due to a marked deterioration in his general condition. His albumin level was 20.1 g/L, and his prealbumin level was 0.10 g/L. After initially refusing enteral nutrition, the patient agreed to a gastrostomy. This was performed on March 12, 2021, under antibiotic prophylaxis with cefazolin. PD was interrupted for 15 days and temporarily replaced by hemodialysis (HD). PD was gradually resumed on March 23, 2021, without complications. Upon discharge on April 7, 2021, the prescription included administering 1 L of nutritional solution (Mégaréal®) per night. The patient weighed 77.2 kg and had albumin at 25.8 g/L and prealbumin at 0.30 g/L.
On June 20, 2021, the gastrostomy tube was accidentally removed. It was reinserted on July 20 (in the absence of the surgeon) with the same antibiotic prophylaxis. Enteral feeding was resumed on July 22 at a rate of 500 mL/day of Mégaréal®. PD was interrupted postoperatively for 15 days in favour of temporary HD.
On September 14, 2021, the patient's weight was 83.7 kg, albuminemia was 33 g/L, and prealbumin was 0.35 g/L. Nutrition was provided by 1 L/day of Mégaréal® and one oral supplement per day.
Figure 1 summarizes the variation in weight and albumin over time.
Figure 1.Variation in weight and albumin over time (years 2020-2021). SNG: nasogastric tube
The patient died suddenly at home on September 18, 2021.
Throughout enteral feeding via the gastrostomy tube, we observed no peritoneal infectious complications, emergence of peritoneal fluid, or dialysate leaks.
Discussion
Gastrostomy is an option for prolonged enteral nutrition when oral nutritional supplements and a nasogastric tube are insufficient or poorly tolerated. In the context of peritoneal dialysis, however, its use remains limited by the fear of peritoneal infections or catheter dysfunction.
In our case, two gastrostomies were performed without any infectious complications related to PD, confirming the technique's feasibility with appropriate preparation and antibiotic prophylaxis.
Nutritional status improved, although this was limited by the patient’s comorbidities and chronic alcoholism. The subsequent cardiac arrest was not attributable to the gastrostomy.
Pediatric experience with gastrostomy in patients with PD dates back more than 25 years.
The first experience reported by Ramage [1] involved 23 children with an average age of 3.8 years. Although the placement of gastrostomy tubes in children treated with PD was accompanied by episodes of peritonitis and wound infection, the experience was considered positive, and gastrostomy feeding in these children was not considered contraindicated, even if it was risky. Indeed, the nutritional benefit outweighs the risk of infection.
In the 2000s, infectious complications mainly consisted of fungal peritonitis, reported in approximately 3% of peritonitis episodes in Warady's study [2]. However, statistically, there was no significant relationship between the presence of a gastrostomy and fungal peritonitis. The authors consider that the presence of a gastrostomy did not appear to be a predisposing factor for the infection, which was treated with antifungal therapy and removal of the peritoneal catheter.
In a more recent study of eight children with an average weight of 6.7 kg treated with PD who underwent percutaneous endoscopic gastrostomy (PEG), prophylactic antibiotic and antifungal treatment for 4 to 5 days reduced the risk of peritonitis. At the same time, PD was resumed 6 days after the gastrostomy was performed. Antibiotic prophylaxis consisted of cefotaxime, and antifungal prophylaxis was performed with echinocandins (caspofungin) [3].
In another study in New Zealand, no cases of fungal peritonitis were reported in 17 children, and there was no significant difference in the incidence of bacterial peritoneal infection before or after gastrostomy tube placement in children treated with PD. It should be noted that the children received antibiotic, but not antifungal, prophylaxis [4].
A retrospective study conducted by Dorman in 23 children with an average age of 1.3 years who underwent laparoscopic gastrostomy confirmed the safety of gastrostomy placement with antibiotic and antifungal prophylaxis, with 0.35 episodes of peritonitis per patient per year after gastrostomy versus 0.45 episodes per patient per year before gastrostomy. It was concluded that laparoscopic gastrostomy is effective, with a safety profile similar to that of surgical gastrostomy, while allowing a clear improvement in these children's nutritional status [5].
Another study compared the simultaneous placement of a laparoscopy-assisted endoscopic gastrostomy and a peritoneal dialysis catheter with the placement of this peritoneal catheter and surgical gastrostomy in 10 patients. There was no significant difference between the two groups in the incidence of bacterial or fungal peritoneal infection or in the survival of the peritoneal catheter in children with a mean age of 5 years [6].
In a study covering 20 years between 2000 and 2020, which included seven children with an average age of 30 months, no significant difference was noted in the risk of peritoneal infection before and after gastrostomy placement, with a peritonitis rate of 0.2/patient/year under intraoperative antibiotic and antifungal prophylaxis and an average duration of gastrostomy and PD use of 18 months. The PD protocol was modified during the perioperative period, with a reduction in the number of bags and a return to the usual protocol 6 days later [7].
The American Society for Gastrointestinal Endoscopy recommends administering cefazolin as antibiotic prophylaxis before each endoscopy with percutaneous endoscopic gastrostomy placement [8].
To our knowledge, no studies on gastrostomy in adult patients with PD have been reported; only a few clinical cases have been described with major complications, particularly infections [9].
Our observation illustrates the feasibility of gastrostomy in adult patients treated with PD, subject to the implementation of antibiotic and antifungal prophylaxis and compliance with a postoperative healing period to avoid leaks, displacement, and infections. This technique ensures effective refeeding in malnourished PD patients with a serious life-threatening prognosis related to malnutrition and after failure of oral supplementation.
Conclusion
Gastrostomy is a safe and effective option for enteral nutrition in peritoneal dialysis patients, provided that a rigorous protocol is followed, including antibiotic and antifungal prophylaxis and temporary interruption of PD.
This observation confirms the absence of major infectious complications and the benefit of gastrostomy in cases of severe malnutrition that are refractory to other nutritional therapeutic approaches.
Authors' Contributions:
JS: wrote the article, MG helped write and proofread the article, RA provided project supervision, and final approval of the version to be published.
Funfing
The authors received no funding for this article
Conflicts of interest
The authors declare no conflict of interest with this article.
Ethical consideration and patient approval
This case report is presented in a fully anonymized form. As the patient was deceased and no identifiable information is disclosed, informed consent was not required according to current ethical guidelines.
ORCID iDs
Justine Schricke : https://orcid.org/0009-0007-1128-7149
Manon Geeraert : https://orcid.org/0009-0000-3160-3283
Raymond Azar : https://orcid.org/0000-0003-0695-1002
References
- Ramage I.J., Harvey E., Geary D.F., Hébert D., Balfe J.A., Balfe J.W.. Complications of gastrostomy feeding in children receiving peritoneal dialysis. Pediatr Nephrol. 1999; Apr;13(3):249-252DOI
- Warady B.A., Bashir M., Donaldson L.A.. Fungal peritonitis in children receiving peritoneal dialysis : a report of the NAPRTCS. Kidney Intern. 2000; 48:384-389. DOI
- Caroline Kempf, J Holle, S Berns, Al Perit Dial Intern 2022. 482-488. DOI
- Prestidge C., Ronaldson J., Wong W., Al Pediatr Nephrol DOI 10.1007/s00467-014-2951-z. DOI
- Dorman M., Benedict L.A., Sujka J., Al Safety of Laparoscopic Gastrostomy in Children Receiving Peritoneal Dialysis. J Surgical Res. 2019;244460-467. DOI
- Lindley R.M., Williams A.R., Fraser N., Al Synchronous laparscopic-assisted percutaneous endoscopic gastrotomy and peritoneal dialysis catheter placement is a valid alternative to open surgery. J Pediat Urol. 2012; 8:527-530. DOI
- Fati F., Pulvirenti R., Longo G.. Percutaneous endoscopic gastrostomy in children receiving peritoneal dialysis : a tertiary centre long-term experience and literature review. Perit Dial Intern. 2024; 44:374-379. DOI
- Antibiotic prophylaxis for GI endoscopy. Gastrointest Endosc. 2015;8181-89. DOI
- Castrale C., Azar R., Piquet M.A., Lobbedez T.. Les spécificités du soin nutritionnel en dialyse péritonéale [The specific nutritionnal care in peritoneal dialysis. Nephrol Ther. 2016. DOI
References
1 - Ramage IJ, Harvey E, Geary DF, Hébert D, Balfe JA, Balfe JW. Complications of gastrostomy feeding in children receiving peritoneal dialysis. Pediatr Nephrol. 1999 Apr;13(3):249-252. doi: https://doi.org/10.1007/s004670050603
2 – Warady BA, Bashir M, Donaldson LA. Fungal peritonitis in children receiving peritoneal dialysis : a report of the NAPRTCS. Kidney Intern 2000 ; 48 : 384-389. doi: https://doi.org/10.1046/j.1523-1755.2000.00176.x
3 - Kempf Caroline, Holle J, Berns S, et Al. Feasibility of percutaneous endoscopic gastrostomy insertion in children receiving peritoneal dialysis. Perit Dial Intern 2022 ; 42 : 482-488. doi: https://doi.org/10.1177/08968608211057651
4 - Prestidge C, Ronaldson J, Wong W, et Al. Infectious outcomes following gastrostomy in children receiving peritoneal dialysis. Pediatr Nephrol DOI 10.1007/s00467-014-2951-z. doi: https://doi.org/10.1007/s00467-014-2951-z
5 - Dorman M, Benedict LA, Sujka J, et Al. Safety of Laparoscopic Gastrostomy in Children Receiving Peritoneal Dialysis. J Surgical Res. 2019 ; 244 : 460-467. doi: https://doi.org/10.1016/j.jss.2019.06.090
6 - Lindley RM, Williams AR, Fraser N, et Al. Synchronous laparscopic-assisted percutaneous endoscopic gastrotomy and peritoneal dialysis catheter placement is a valid alternative to open surgery. J Pediat Urol 2012 ; 8 : 527-530. doi: https://doi.org/10.1016/j.jpurol.2011.09.011
7- Fati F, Pulvirenti R, Longo G, et al. Percutaneous endoscopic gastrostomy in children receiving peritoneal dialysis : a tertiary centre long-term experience and literature review. Perit Dial Intern. 2024 ; 44 : 374-379. doi: https://doi.org/10.1177/08968608231223812
8 - ASGE Standards of Practice Committee; Khashab MA, Chithadi KV, Acosta RD, Bruining DH, Chandrasekhara V, Eloubeidi MA, Fanelli RD, Faulx AL, Fonkalsrud L, Lightdale JR, Muthusamy VR, Pasha SF, Saltzman JR, Shaukat A, Wang A, Cash BD. Antibiotic prophylaxis for GI endoscopy. Gastrointest Endosc. 2015 ; 81 : 81-89. doi: https://doi.org/10.1016/j.gie.2014.08.008
9 - Castrale C, Azar R, Piquet MA, Lobbedez T. Les spécificités du soin nutritionnel en dialyse péritonéale [The specific nutritionnal care in peritoneal dialysis]. Nephrol Ther. 2016 Jul;12(4):198-205. French. doi: https://doi.org/10.1016/j.nephro.2016.03.004. Epub 2016 Jun 16. PMID: 27320370.
Downloads
Submitted
Accepted
Published
How to Cite
Issue
Section
License
Copyright (c) 2025 Justine SCHRICKE

This work is licensed under a Creative Commons Attribution 4.0 International License.








