Current status of bariatric surgery treatment in peritoneal dialysis
DOI:
https://doi.org/10.25796/bdd.v8i4.87096Keywords:
peritoneal dialysis, bariatric surgery, obesityAbstract
Obesity is a major public health issue that affects a significant proportion of patients with end-stage renal disease (ESRD). In patients undergoing peritoneal dialysis (PD), obesity complicates treatment by increasing the risk of mechanical complications and infections and reducing the effectiveness of peritoneal exchanges. Furthermore, obesity limits access to kidney transplantation, making weight loss a crucial goal. Bariatric surgery is emerging as an effective strategy for improving metabolic condition and promoting placement on a transplant waiting list.
Sleeve gastrectomy (SG) is now the preferred technique for helping obese patients on ESRD lose weight, particularly due to its favorable safety profile, reduced operating time, and absence of intestinal bypass, thus limiting the risk of deficiencies. The available data, although limited to case series and isolated reports, suggest that SG can be performed in PD patients either with early resumption of PD or after a temporary transition to hemodialysis depending on clinical status. Optimized protocols include a gradual resumption of PD at low volumes, minimizing the risk of leakage or infection.
Bariatric surgery therefore appears feasible and generally safe in PD patients, provided that a rigorous multidisciplinary assessment and close nutritional monitoring are carried out to prevent malnutrition and sarcopenia. It is a relevant therapeutic option for improving access to kidney transplantation and optimizing the prognosis of obese patients with ESRD. This article was written following a presentation at the Société Francophone de Néphrologie, Dialyse et Transplantation 2025 on the feasibility of bariatric surgery in PD.
Introduction
Obesity is a major public health challenge, affecting nearly 40% of adults in the United States, with an identical prevalence reported in the population with end-stage chronic kidney disease (ESCKD) 12.
In France, nearly one in two adults is overweight (overweight or obese), with approximately 17% of these adults being obese (body mass index [BMI] ≥ 30 kg/m²) and 2% being severely obese (BMI index ≥ 40 kg/m²) 3. The prevalence of obesity is also high among patients with ESCKD; according to data from the REIN registry, approximately 23% of men and 31% of women are obese (BMI > 30) at the start of replacement therapy (dialysis or transplant). Obesity is recognized as an aggravating factor in chronic kidney disease and contributes to progression to end-stage renal disease (ESRD). In addition, it complicates the management of dialysis patients (for example, by increasing technical difficulties and certain risks in peritoneal dialysis [PD]). Therefore, optimizing weight management at all stages of kidney disease is essential.
In ESRD patients, obesity is a factor that limits a patient's access to kidney transplantation and is associated with poorer post-transplant outcomes, including poorer kidney graft survival and excess mortality 456. Bariatric surgery is increasingly recognized as an effective strategy for inducing significant weight loss, improving associated comorbidities (such as diabetes), and facilitating access to transplant lists. In the latest Haute Autorité de Santé (HAS; French National Authority for Health) recommendations in 2024, chronic kidney disease is an integral part of the indications for bariatric surgery for:
- patients with a BMI between 35 and 40 kg/m² associated with chronic kidney disease (up to moderate CRF: stages 3A and 3B) after consultation with a nephrologist
- patients with a BMI between 35 and 40 kg/m², severe, or end-stage renal failure with plans for transplantation.
This second indication must be discussed on a case-by-case basis with the transplant team due to the risk of morbidity and mortality and must be performed in centers with dialysis facilities.
Obesity and peritoneal dialysis: challenges and impacts
Obesity poses several challenges for patients on PD. From a pathophysiological perspective, excess adipose tissue can limit the effectiveness of peritoneal exchanges (due to an increased weight/body surface area ratio) and increase intra-abdominal pressure, promoting the onset of mechanical complications such as hernias or dialysate leaks. In addition, studies suggest that obesity may increase the risk of peritonitis in PD. A meta-analysis of 18 studies showed a significantly higher incidence of peritonitis in obese patients on PD than in normal-weight patients 7.
Indications for bariatric surgery in peritoneal dialysis patients
The indication for bariatric surgery in obese dialysis patients (whether PD or hemodialysis [HD]) is based on several prognostic considerations. First, severe obesity limits access to kidney transplantation. Many centers set a BMI threshold (often 35 kg/m²) for patients; if patients exceed this threshold, their registration on the transplant waiting list is restricted. Bariatric surgery therefore acts as a "bridge" to transplantation; by inducing significant weight loss, it brings the patient's BMI below the thresholds required for transplantation, thereby increasing their chances of receiving a kidney transplant.
In addition to improving the prospect of transplantation, bariatric surgery aims to improve the overall health of obese dialysis patients. It can reduce obesity-related comorbidities (type 2 diabetes, hypertension, sleep apnea, etc.), which is particularly beneficial in patients whose functional reserve is already diminished by ESRD. Thus, bariatric surgery can improve the prognosis of obese dialysis patients by increasing their eligibility for transplantation and reducing obesity-related complications.
However, candidates must be rigorously selected and optimally prepared. HAS recommendations advocate a thorough multidisciplinary assessment before any bariatric surgery, particularly in patients with renal failure, who are "fragile" patients. A multidisciplinary consultation (RCP) involving nephrologists, bariatric surgeons, anesthetists-resuscitators, dieticians, psychologists, and other specialists is essential to weigh the benefits and risks of the process and to ensure the patient's motivation and ability to adhere to long-term follow-up. The objectives of the surgery must be clearly defined: improving the patient's condition with a view to transplantation, but also maintaining quality of life on dialysis while awaiting the transplant, with close nutritional monitoring to prevent malnutrition after surgery.
Different bariatric surgery techniques
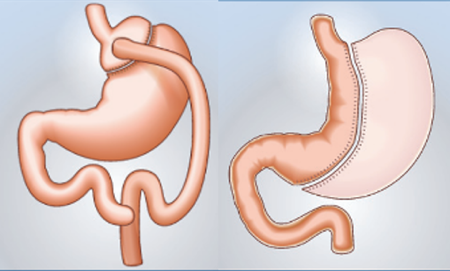
Between 2006 and 2016, the number of patients with ESKD undergoing bariatric surgery increased ninefold in the United States 1. This decade also saw a change in surgical practices: sleeve gastrectomy (SG) became the procedure of choice, ahead of Roux-en-Y gastric bypass. This change also reflects trends observed in the general population without ESKD in the United States. In France, according to DREESS data, the number of bariatric surgeries has also been steadily increasing in recent years, with SG also being the most commonly performed procedure 3 (Figure 1).
Figure 1.Different bariatric surgery techniques – HAS 2009.
| Criteria | Sleeve Gastrectomy | Bypass (Roux-en-Y) |
| Weight loss (1–2 years) |
Significant – ~50%–60% in 2 years (mainly restrictive technique) |
Very significant – ~70%–80% in 2–3 years maximum result (associated malabsorptive element) |
| Disadvantages for patients with PD |
Risk of GERD, irreversible, possible gastric leakage |
More complex technique, risk of anastomotic ulcers, rupture of digestive continuity, greater number of trocars, higher risk of malnutrition |
HAS recommends several bariatric surgery procedures, which are summarized in Table I.Among these, gastric bypass offers the most significant weight loss and the best remission of metabolic disorders associated with obesity (type 2 diabetes, high blood pressure, dyslipidemia, sleep apnea syndrome) 9, but this comes at the cost of increased technical complexity and a higher risk of metabolic complications (hyperoxaluria, malabsorption) and nutritional deficiencies. SG (longitudinal gastrectomy) is an interesting compromise, allowing for significant weight loss and improvement in metabolic disorders while causing fewer serious complications than a bypass. However, it is contraindicated in cases of esophageal reflux. Endosleeve is an endoscopic technique that is less effective than conventional SG and is currently reserved for patients with contraindications to surgery. Adjustable gastric banding is the least invasive procedure, but also the least effective in terms of weight loss and metabolic benefits (modest weight loss, limited metabolic benefit).
From a technical standpoint, laparoscopic SG (longitudinal gastrectomy) is often preferred for these patients. This bariatric surgery technique involves removing about two-thirds of the stomach (mainly the fundic portion that secretes ghrelin) to reduce gastric capacity and appetite without performing intestinal bypass. SG has several advantages in the context of CRF: It is generally shorter and less complex than a bypass, does not cause malabsorption (which limits the risk of nutritional deficiencies in already vulnerable patients), often requires fewer trocars, and has a lower risk of anastomotic fistula because there is no digestive anastomosis. In addition, the presence of a PD catheter with adhesions can make bypass surgery more difficult. In the literature, SG is described as a safe and effective option in obese dialysis patients, allowing them to achieve a BMI that is compatible with transplantation while minimizing operative morbidity.
Certain tools are used to estimate weight loss based on the patient's clinical data and the type of surgery planned 10. Performing such surgeries in patients on PD raises specific concerns, including risk of infection, dialysate leaks, peritoneal membrane failure, and the question of whether PD should be continued versus temporarily switching to HD.
For obese patients on PD, bariatric surgery also presents a unique challenge: avoiding a temporary transition to HD, which would involve the risk of complications related to central venous catheters and infections.
Perioperative management and feasibility in peritoneal dialysis
A Canadian case series published in 2025 11 retrospectively reported on 11 PD patients who underwent SG to be eligible for kidney transplantation. In this study, all patients systematically underwent dialysis catheter placement prior to surgery with a temporary switch to HD during the postoperative healing period. These results confirmed the effectiveness of SG in PD patients, with a median BMI decreasing from 41 kg/m² to 31 mg/m². Eighty percent of patients were able to resume PD after a median of 6 weeks of transition to HD, and 70% of patients had access to a kidney transplant during the follow-up period. In terms of infectious complications, there was one case of HD catheter bacteremia and one case of PD fluid infection upon resumption of PD, requiring a catheter change.
In 12 reported the cases of five patients who underwent gastric banding or bypass surgery with resumption of PD postoperatively. Several case reports were subsequently published by 13, Nguyen et al. in 2020 14, and van Diepen in 2023 15, each involving laparoscopic SG surgery with early resumption of PD without switching to HD. In all cases, these procedures led to a rapid improvement in BMI, with a moderate risk of leaks and infectious complications. The PD management protocol was often similar: fasting on the day of surgery, resumption of automated PD on a cycler from day 1 with short cycles (often 1 to 2 hours) at low volume (often 1 L), fasting during the day for 2 weeks, then gradual increase until resumption of the usual protocol, most often at 4 weeks. This protocol therefore seems particularly feasible in patients with preserved diuresis. In anuric patients, the strategy reported by Ali Karam et al. 11, i.e., the temporary placement of a tunneled catheter for a period of HD before resuming PD after healing, should be discussed.
The postoperative period should be an opportunity for close monitoring to allow treatments to be adjusted, particularly antihypertensive drugs and the dialysis schedule, following changes induced by the surgery (improved blood pressure, reduced food intake, etc.).
A crucial aspect of post-bariatric surgery follow-up in these patients is ensuring that they maintain proper nutritional status. Dialysis, and PD in particular, exposes patients to protein loss and a risk of malnutrition, which can be exacerbated by post-surgical dietary restrictions. Therefore, patients need to receive support from a dietary team to adjust their protein and calorie intake, supplement vitamins and trace elements if necessary, and monitor albumin levels and muscle condition. The goal should therefore be controlled weight loss, with close monitoring, to bring the patient to transplantation in the best possible condition without compromising their functional reserve through malnutrition.
In cases where the goal is moderate weight loss, other less invasive techniques should be considered, such as endosleeve or medical treatment with GLP-1 analogues.
A few relevant case reports have been published 16 and seem to show a good safety profile with effective weight loss. These treatments can also be used either as an alternative for patients who are not eligible for surgery or as a supplement to stabilize postoperative weight loss. However, their prescription for dialysised patients is not currently recommended due to very limited experience in these patients.
Conclusion
The epidemiology of obesity in France, both in the general population and in patients with ESRD, fully justifies the attention paid to this issue. Obesity affects a growing proportion of dialysis patients, with negative implications for their access to transplantation and possibly for the progression of their kidney disease.
Bariatric surgery appears to be possible and safe in patients on PD, provided there is close collaboration between nephrologists, bariatric surgeons, and PD teams. SG currently appears to be the best option; it is effective for weight loss, technically feasible by laparoscopy, and compatible with the continuation or rapid resumption of PD without systematic recourse to HD. It enables significant weight loss, thereby improving patients’ eligibility for kidney transplantation and, as a result, ensuring long-term survival compared to continuing dialysis without surgery. The risk of postoperative and infectious complications appears to be comparable to that of the general population. However, particular attention should be paid to the risk of sarcopenia in these patients. Each case must, of course, be assessed individually in a multidisciplinary team meeting, taking into account the patient’s surgical risk profile, nutritional status, social support, and motivation. The success of this approach relies on integrated multidisciplinary care, entailing collaboration between nephrologists, bariatric surgeons, nutritionists, and other specialists throughout the pre- and postoperative process. The main objective is still to ensure access to kidney transplantation, but these interventions can also improve metabolic control, quality of life, and dialysis tolerance.
Authors’ Contributions
VF and GB wrote the article, and MFE CL and TL reviewed the article and provided comments and corrections.
Ethical Considerations and Patient Consent
Not applicable
Data Availabilité Statement
Not applicable
Funding
The authors did not receive any funding for this article
Conflicts of Interest
The authors declare no conflicts of interest.
ORCID iDs
Victor Fages : https://orcid.org/0000-0002-1722-9042
Grégory Baud : https://orcid.org/0000-0001-7130-7315
Marion Féricot : https://orcid.org/0009-0009-1006-2225
Célia Lessore : https://orcid.org/0009-0008-9767-5704
Thierry Lobbedez : https://orcid.org/0000-0003-2914-6786
References
- Sheetz K.H., Gerhardinger L., Dimick J.B., Waits S.A.. Bariatric Surgery and Long-term Survival in Patients With Obesity and End-stage Kidney Disease. JAMA Surg. 2020. DOI
- MD1,2. Population-based Trends in Obesity and Kidney Transplantation Among Patients With End-stage Kidney Disease. Transplantation Direct. 2021; 7(12)DOI
- Fontbonne A., Currie A., Tounian P., Picot M.C., Foulatier O., Nedelcu M., Nocca D.. Prevalence of Overweight and Obesity in France: The 2020 Obepi-Roche Study by the «Ligue Contre l’Obésité». J Clin Med. 2023. DOI
- Segev D.L., Simpkins C.E., Thompson R.E., Locke J.E., Warren D.S., Montgomery R.A.. Obesity impacts access to kidney transplantation. J Am Soc Nephrol. 2008; Feb;19(2):349-55DOI
- Hill C.J., Courtney A.E., Cardwell C.R., Maxwell A.P., Lucarelli G., Veroux M., Furriel F., Cannon R.M., Hoogeveen E.K., Doshi M., McCaughan J.A.. Recipient obesity and outcomes after kidney transplantation: a systematic review and meta-analysis. Nephrol Dial Transplant. 2015; Aug;30(8):1403-11DOI
- Meier-Kriesche H.U., Arndorfer J.A., Kaplan B.. The impact of body mass index on renal transplant outcomes: a significant independent risk factor for graft failure and patient death. Transplantation. 2002. DOI
- Chen X., Mao Y., Ge Y.. Does Body Mass Index Impact the Outcomes of Peritoneal Dialysis Patients? A Systematic Review and Meta-Analysis of Non-Randomized Trials. Obes Facts. 2025. DOI
- P Courcoulas A., R Daigle C., E Arterburn D.. Long term outcomes of metabolic/bariatric surgery in adults BMJ. 2023. DOI
- Queiroz S., Gadelha J.G., Husain N., Gutu C.S.. Effect of Gastric Bypass vs Sleeve Gastrectomy on Remission of Type 2 Diabetes Mellitus Among Patients with Severe Obesity: A Meta-Analysis. Obes Surg. 2025. DOI
- Development and validation of an interpretable machine learning-based calculator for predicting 5-year weight trajectories after bariatric surgery: a multinational retrospective cohort SOPHIA study. Saux, Patrick et al.The Lancet Digital Health. 5(ue 10):692-702.
- Karam A.A., Safar A., Vourtzoumis P., Demyttenaere S., Court O., Andalib A.. How to manage peritoneal dialysis in patients undergoing bariatric surgery? A case series from a single academic center. Surg Endosc. 2025. DOI
- Valle G.A., Kissane B.E., Cruz-Muñoz N.. Successful laparoscopic bariatric surgery in peritoneal dialysis patients without interruption of their CKD6 treatment modality. Adv Perit Dial. 2012; 28(134-9)
- Imam T.H., Wang J., Khayat F.S.. Bariatric Surgery in a Patient on Peritoneal Dialysis. Peritoneal dialysis international : journal of the International Society for Peritoneal Dialysis. 2013; 33:710-711.
- Nguyen A.H., Naljayan M., Yazdi F., Reisin E.. Laparoscopic Sleeve Gastrectomy in a Patient on Peritoneal Dialysis. Kidney Int Rep. 2020; 3;5(12):2361-2364DOI
- Diepen ATW Vening, Meulen RCJC Verhave. Avoiding interim haemodialysis by early restart of peritoneal dialysis following sleeve gastrectomy for obesity. Peritoneal Dialysis International: Journal of the International Society for Peritoneal Dialysis. 2023; 43(4):345-347. DOI
- Yavorskiy P., Borrelli S., Esposito K., Maiorino M.I., Petrizzo M., Nicola L., Garofalo C.. Lesson for the clinical nephrologist: glucagon-like peptide-1 receptor agonists (GLP-1 RA) in a patient with obesity and diabetic kidney disease on peritoneal dialysis. J Nephrol. 2025. DOI
References
1) Sheetz KH, Gerhardinger L, Dimick JB, Waits SA. Bariatric Surgery and Long-term Survival in Patients With Obesity and End-stage Kidney Disease. JAMA Surg. 2020 Jul 1;155(7):581-588. doi: https://doi.org/10.1001/jamasurg.2020.0829.
2) Wakam, Glenn K. MD1; Sheetz, Kyle H. MD, MS1,2; Gerhardinger, Laura MA2; Montgomery, John R. MD1,2; Waits, Seth A. MD1,2. Population-based Trends in Obesity and Kidney Transplantation Among Patients With End-stage Kidney Disease. Transplantation Direct 7(12):p e787, December 2021. | doi: https://doi.org/10.1097/txd.0000000000001163
3) Fontbonne A, Currie A, Tounian P, Picot MC, Foulatier O, Nedelcu M, Nocca D. Prevalence of Overweight and Obesity in France: The 2020 Obepi-Roche Study by the «Ligue Contre l’Obésité». J Clin Med. 2023 Jan 25;12(3):925. doi: https://doi.org/10.3390/jcm12030925.
4) Segev DL, Simpkins CE, Thompson RE, Locke JE, Warren DS, Montgomery RA. Obesity impacts access to kidney transplantation. J Am Soc Nephrol. 2008 Feb;19(2):349-55. doi: https://doi.org/10.1681/asn.2007050610. Epub 2007 Dec 19.
5) Hill CJ, Courtney AE, Cardwell CR, Maxwell AP, Lucarelli G, Veroux M, Furriel F, Cannon RM, Hoogeveen EK, Doshi M, McCaughan JA. Recipient obesity and outcomes after kidney transplantation: a systematic review and meta-analysis. Nephrol Dial Transplant. 2015 Aug;30(8):1403-11. doi: https://doi.org/10.1093/ndt/gfv214. Epub 2015 Jun 4.
6) Meier-Kriesche HU, Arndorfer JA, Kaplan B. The impact of body mass index on renal transplant outcomes: a significant independent risk factor for graft failure and patient death. Transplantation. 2002 Jan 15;73(1):70-4. doi: https://doi.org/10.1097/00007890-200201150-00013.
7) Chen X, Mao Y, Ge Y. Does Body Mass Index Impact the Outcomes of Peritoneal Dialysis Patients? A Systematic Review and Meta-Analysis of Non-Randomized Trials. Obes Facts. 2025 Oct 16:1-20. doi: https://doi.org/10.1159/000548725. Epub ahead of print.
8) Courcoulas A P, Daigle C R, Arterburn D E. Long term outcomes of metabolic/bariatric surgery in adults BMJ 2023; 383 :e071027 doi: https://doi.org/10.1136/bmj-2022-071027
9) Queiroz S, Gadelha JG, Husain N, Gutu CS. Effect of Gastric Bypass vs Sleeve Gastrectomy on Remission of Type 2 Diabetes Mellitus Among Patients with Severe Obesity: A Meta-Analysis. Obes Surg. 2025 Jun;35(6):2296-2302. doi: https://doi.org/10.1007/s11695-025-07858-w. Epub 2025 May 16.
10) Development and validation of an interpretable machine learning-based calculator for predicting 5-year weight trajectories after bariatric surgery: a multinational retrospective cohort SOPHIA study. Saux, Patrick et al.The Lancet Digital Health, Volume 5, Issue 10, e692 - e702
11) Karam AA, Safar A, Vourtzoumis P, Demyttenaere S, Court O, Andalib A. How to manage peritoneal dialysis in patients undergoing bariatric surgery? A case series from a single academic center. Surg Endosc. 2025 Jul;39(7):4479-4485. doi: https://doi.org/10.1007/s00464-025-11800-7. Epub 2025 May 19. PMID: 40389654.
12) Valle GA, Kissane BE, de la Cruz-Muñoz N. Successful laparoscopic bariatric surgery in peritoneal dialysis patients without interruption of their CKD6 treatment modality. Adv Perit Dial. 2012;28:134-9. PMID: 23311230.
13) Imam, T.H. & Wang, J & Khayat, F.S.. (2013). Bariatric Surgery in a Patient on Peritoneal Dialysis. Peritoneal dialysis international : journal of the International Society for Peritoneal Dialysis. 33. 710-711. 10.3747/pdi.2012.00272.
14) Nguyen AH, Naljayan M, Yazdi F, Reisin E. Laparoscopic Sleeve Gastrectomy in a Patient on Peritoneal Dialysis. Kidney Int Rep. 2020 Oct 3;5(12):2361-2364. doi: https://doi.org/10.1016/j.ekir.2020.09.014. PMID: 33305132; PMCID: PMC7710827.
15) Van Diepen AT, Vening W, ter Meulen RC, Verhave JC. Avoiding interim haemodialysis by early restart of peritoneal dialysis following sleeve gastrectomy for obesity. Peritoneal Dialysis International: Journal of the International Society for Peritoneal Dialysis. 2023;43(4):345-347. doi: https://doi.org/10.1177/08968608231167246
16) Yavorskiy P, Borrelli S, Esposito K, Maiorino MI, Petrizzo M, De Nicola L, Garofalo C. Lesson for the clinical nephrologist: glucagon-like peptide-1 receptor agonists (GLP-1 RA) in a patient with obesity and diabetic kidney disease on peritoneal dialysis. J Nephrol. 2025 Apr;38(3):1123-1125. doi: https://doi.org/10.1007/s40620-025-02247-z.
Downloads
Submitted
Accepted
Published
How to Cite
Issue
Section
License
Copyright (c) 2025 Victor Fages, Gregory Baud, Marion Fericot, Célia LESSORE, Thierry Lobbedez

This work is licensed under a Creative Commons Attribution 4.0 International License.








